BIOLIGHT TOXY
Luminometer for bioluminescent toxicity testing
The BioLight Toxy luminometer offers the most up to date dual option instrument for bioluminescent toxicty testing with Aliivibrio fischeri (formerly named Vibrio fischeri) in the market. This is a result of matching a team of experts in ecotoxicity to fulfill the needs of toxicity users around the world.
This state-of-the-art luminometer brings together the capabilities of doing portable testing in the field as well as bench top testing in the lab. The BioLight Toxy all-in-one unit is designed to be used in conjuction with the BioLight Multi and Single Reagent as well as BioLight Recon, BioLight Diluent and BioLight Salinity Adjusting Solution.


BioLight Toxy Brings Rapid Toxicity Testing To The 21st Century
VERSATILE
Modern
AFFORDABLE

◊ Versatile modular bench-top and portable reader in one
◊ Expandable bench-top offers up to 2 cooling block per unit
◊ Customizable easy to use configurable protocols
◊ Reliable meets all international standards such as ISO 11348-3 ;
ISO 21338 ; NMX-AA-1112-SCFI-207
◊ Rapid read time < 7 seconds
◊ Independent no need of tablet or PC
◊ Accessible remote access
◊ Value most cost-effective offering in the industry

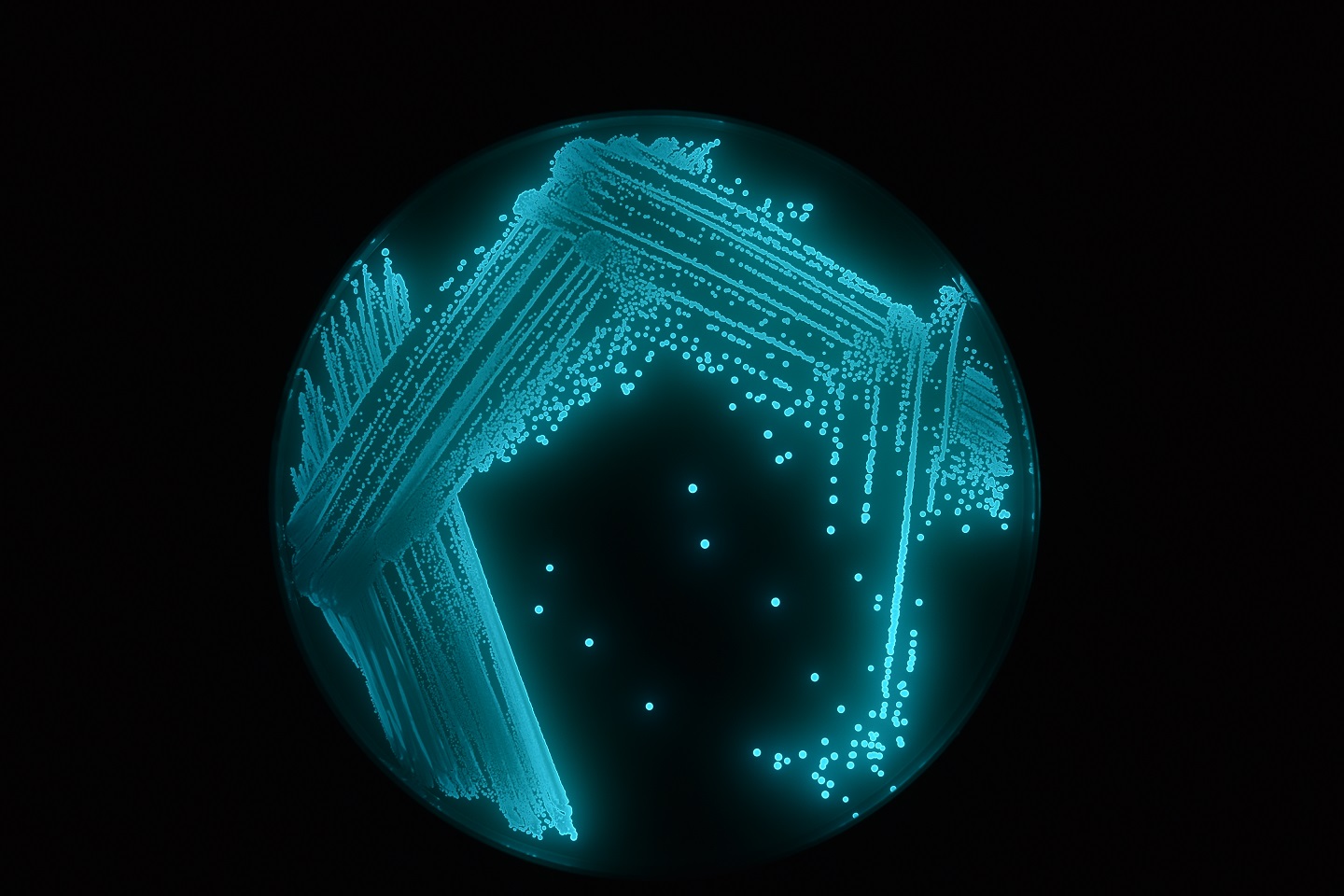
BIOLIGHT Luminescent bacteria test
Aliivibrio fischeri (formerly Vibrio fischeri ) is a marine, Gram negative and non-pathogen bacterium that luminesces as a natural part of its metabolism under optimal environmental conditions.
When exposed to a toxic substance, the metabolic respiratory process of this bioluminescent bacterium is disrupted, reducing light output. This inhibition of luminescence by Aliivibrio fischeri can be measured after 5-30 minutes with a photometer (luminometer).
The reduction of light intensity measured, directly correlates with the degree of toxicity of the sample relative to the control sample.
The application of freeze-dried luminescent bacteria proves to be a useful tool for the assessment and monitoring of toxicity.
Using the BioLight Toxy along with the BioLight Reagents and consumables provides testing that screen over 3.600 chemical compounds simultaneously.
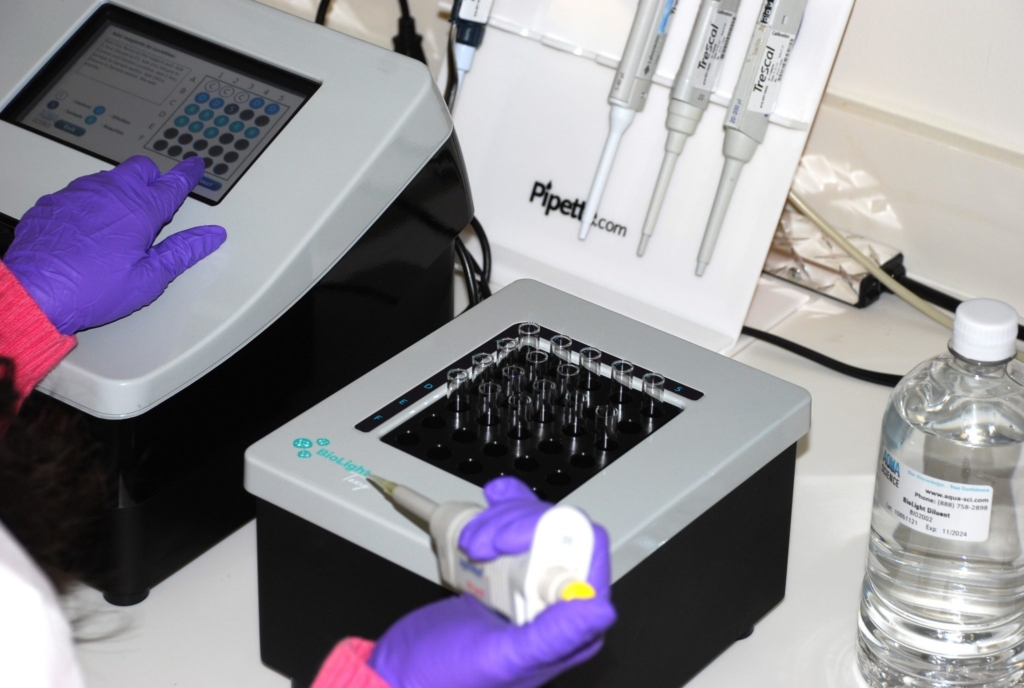
Bioassays using microbial biosensors are rapid, sensitive, reproducible and user-friendly.
BioLight Toxy bench top
The BioLight Toxy – Bench Top instrument includes a cooled read well and cooled reagent well along with a separate cooling block to ensure all samples are kept at the appropriate temperatures during testing. There is an option to add an additional cooling block to double the number of samples that can be tested (up to 60 samples).
Additionally required to perform the analysis are the BioLight Multi Reagent, BioLight Diluent, BioLight Recon, BioLight Salinity Adjusting and disposable glass cuvettes.

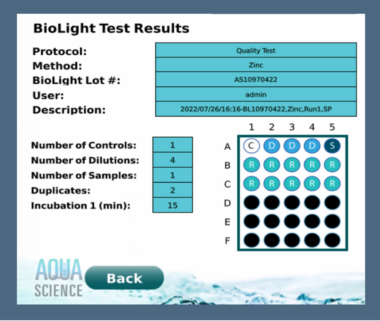
When using the BioLight Toxy – Bench Top, there will be multiple protocols available to choose in the onboard software. The software is easy to use and provides all results calculations that can be printed or uploaded to a computer as well as saved in the software. Choosing a protocol will be based on the water type, expected toxicity level and results of the baseline data if available. Most protocols provide results in approximately 45 minutes, including the 15 minutes for the BioLight Multi reagent to equilibrate after reconstitution.
BioLight Toxy

This test will provide results in 20 minutes total. The Toxy can be set to read again at 15 minutes if required to see if there is a significant difference in the light loss. Different chemicals often have effects on the light change at different times. Light loss may be present at 5 minutes but may continue to decrease at 15 minutes. The 15-minute reading may be more reliable.
BIOLIGHT Toxy Features A Custom PLC Software Interface On-Board
User can either choose from a library of common toxicity tests already pre-programmed in the software or create a fully customized test that can be saved to the library for future testing. This is just one of many helpful features that have been incorporated into this new state-of-the art luminometer, making rapid acute toxicity testing even easier.
PROTOCOLS
EC50 Test 2%, 45% or 81.9%
Testing that provides a calculated EC50 value and measure relative toxicity.
- 2% EC50 Test – samples expected to have a high level of toxicity. Typically, this would be for wastewater influent or pure compounds.
- 45% EC50 Test – samples of unknown toxicity. This EC50 test measures relative toxicity and is the most adaptable. It is a good option for septage, influent wastewater and water from a treatment digester.
- 81.9% EC50 – samples expected to have low to medium toxicity. Primarily used for drinking water, wastewater effluent, pore water and stormwater. The expected EC50 level for this test should be between 30% to 50%.
% Effect Test – 2%, 45% or 81.9%
Testing using a single concentration. This test will not determine relative toxicity or provide a dose response curve.
- 2% Effect Test – Used for samples with high toxicity. Wastewater influent, septage, industrial trucked waste.
- 45% Effect Test – Used for samples of medium toxicity.
- 81.9% Effect Test – Used with samples of low toxicity such as drinking water.
Comparison Test
Used to compare the relative acute toxicity of an undetermined sample with a control sample. A control sample could be a sample of a determined toxicity. This is the best test for the analysis of low toxicity samples if the EC50 values can’t be achieved using the EC50 test.
ISO
Complies with ISO 11348-3. The ISO Standard states that the method is applicable for wastewater, fresh water – both surface and ground, sea and also brackish water, eluates of sediments, pore water – both aqueous extracts and leachates.
Solids Test
Used for soils, sediments and other solid samples. The test uses solid samples which are serial diluted with 9.9% being the highest concentration. It can be used for freshwater, marine and estuarine samples with the appropriate diluent. It allows for the detection of toxicity due to the insoluble solids that are not in solution by allowing the test organisms to come in direct contact with the solid sample in an aqueous suspension.
Order information
- order code BIO2019L BioLight Toxy – Bench Top
Includes : BioLight Instrument with on board PLC and software for both field and lab protocols.
Cooling block to hold 12 mm cuvettes with USB connection to the cooling unit for power - order code BIO2018P BioLight Toxy – Portable
Includes : BioLight Instrument with on board PLC and software for field protocols.
No rechargable battery included*
*can be connected with external portable Power Station or wall plug for inside usage - order code BIO2018A Upgrade from BioLight Portable to Bench Top
Includes : Cooling block to hold 12 mm cuvettes with USB connection to the unit for power.
USB for adding lab protocols to the software - order code BIO2020 Cooling Block for 12 mm Tubes
Includes : Cooling block to hold 12 mm cuvettes with USB connection to the unit for power.
This item can only be acquired when the user has purhased BIO2019L or BIO2018A. - order code BIO2021 Cooling Block for 17 mm Tubes (Solids Testing)
Includes : Cooling block to hold 17 mm cuvettes with USB connection to the unit for power.
This item can only be acquired when the user has purhased BIO2019L or BIO2018A.